Discussion Guide
Evidence-Based Strategies for Reversing Direct-Acting Oral Anticoagulants
Learning Objectives
After completing this knowledge-based educational activity, pharmacists should be able to
- Describe the indications for use, storage, preparation, dosing, and administration of specific pharmacologic reversal agents for direct-acting oral anticoagulants (DOACs)
- Summarize recent clinical evidence of the safety and efficacy of specific pharmacologic reversal agents for DOACs
- Identify authoritative evidence-based protocols and guidelines for using DOAC reversal agents
Relevant Financial Relationship Disclosure and Format
No one in control of the content of this activity has a relevant financial relationship with an ineligible company.
As defined by the Standards of Integrity and Independence definition of ineligible company.
Format
This online activity consists of text, interactive questions, and an evaluation. View the system requirements. CPE information and instructions for processing CE is located at the end of this activity.
Executive Summary
Direct-acting oral anticoagulants (DOACs) were developed to overcome some of the limitations of warfarin, and the use of these newer agents has increased in recent years. However, there is a risk for bleeding during DOAC use, and this risk needs to be weighed against the risk of thrombosis in patients receiving these drugs. DOAC-related bleeding is associated with substantial morbidity and mortality. Anticoagulation from DOACs may need to be reversed before surgery if the need for the surgery is urgent or immediate.
General approaches that could be used to limit continued exposure to a DOAC include withholding the drug, oral administration of activated charcoal, and hemodialysis. Various nonspecific pharmacologic interventions, such as concentrated clotting factor products, that have been used in the past to reverse the anticoagulant effects of warfarin have been used to replenish clotting factors inhibited by DOACs.
The specific pharmacologic reversal agents idarucizumab and andexanet alfa were developed for use in patients with or at risk for bleeding from DOACs. The decision to use these agents may be based on the location and severity of bleeding, urgency of surgery, and quantitative or qualitative laboratory assays for DOAC level. The type of DOAC, dose, time elapsed since it was last taken, and preparation requirements are practical considerations in the use of andexanet alfa.
Guidelines for the use of DOAC reversal agents have been released by authoritative organizations based on currently available clinical data. These guidelines are consistent in their recommendations for the use of specific pharmacologic reversal agents as first-line therapy for DOAC reversal in patients with major or life-threatening bleeding or an urgent need for surgery associated with a high risk of bleeding. To reduce practice variation and optimize patient outcomes, these evidence-based guidelines should be taken into consideration in developing institutional protocols and guidelines for managing patients receiving DOACs who experience major or life-threatening bleeding or have an urgent need for surgery associated with a high risk of bleeding.
Introduction
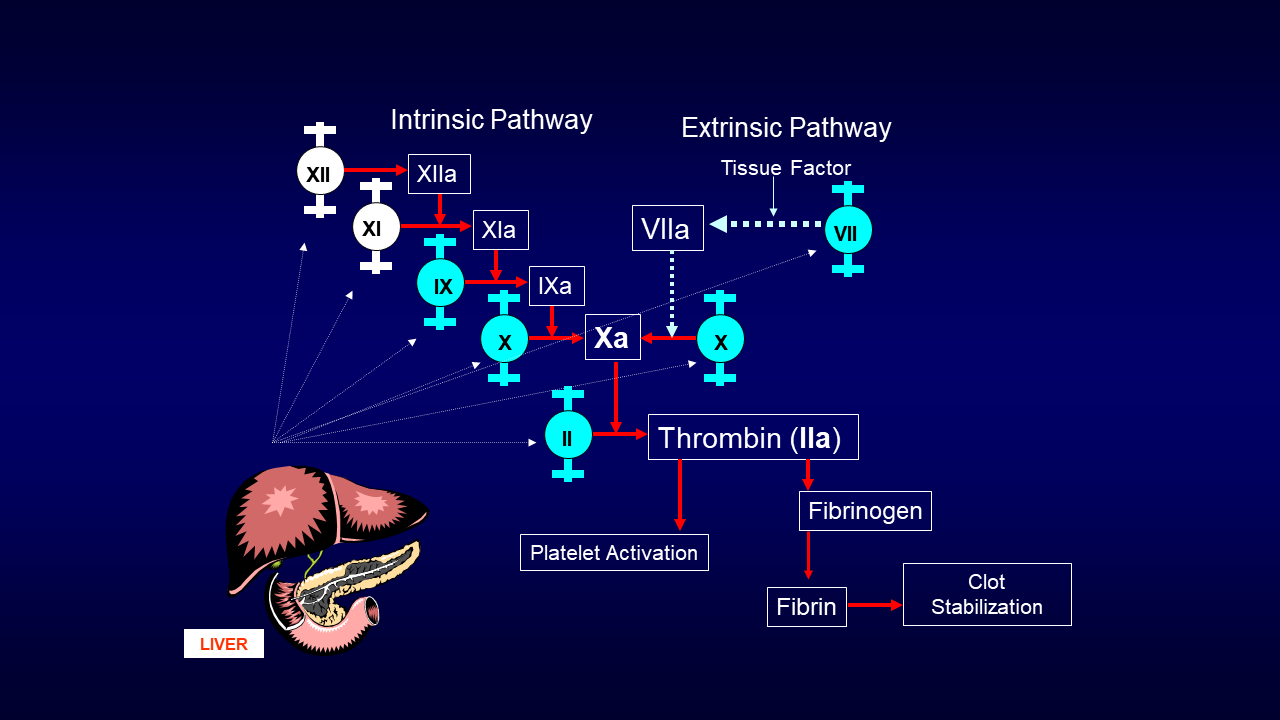
Warfarin has been the mainstay of anticoagulant therapy since the 1950s. It binds the enzyme vitamin K epoxide reductase needed for the conversion of oxidized vitamin K to reduced vitamin K, which is required for the hepatic formation of active clotting factors II, VII, IX, and X. These vitamin K-dependent clotting factors play vital roles in the intrinsic and extrinsic pathways of the clotting cascade (Figure) resulting in the generation of thrombin (factor IIa), conversion of fibrinogen to fibrin, and clot formation and stabilization.1
Figure. Clotting Cascade
Direct-acting oral anticoagulants (DOACs) that act specifically on factor IIa (dabigatran) or factor Xa (rivaroxaban, apixaban, edoxaban, and betrixaban) were developed to overcome the shortcomings of warfarin (betrixaban is no longer available in the United States because of a business decision by the manufacturer to discontinue marketing the drug). The limitations of warfarin include a narrow therapeutic range, need for frequent laboratory monitoring and dosage adjustment to avoid bleeding and ensure antithrombotic efficacy, slow onset and offset of action, and risk for drug-drug and drug-food interactions. The DOACs have favorable risk/benefit profiles compared with warfarin and injectable anticoagulants when used for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation or preventing or treating venous thromboembolism (VTE).2,3 The DOACs have predictable pharmacokinetics (Table 1) with short half-lives, a rapid onset and offset of action, and few drug-food interactions, so frequent laboratory monitoring is not required.8 Fixed dosing is used for DOACs, although individualization of dosing is sometimes needed based on weight, renal function, age, and concomitant medication use (i.e., risk of drug-drug interactions). Advanced age is associated with renal impairment affecting the disposition of most DOACs.9
Table 1. DOAC Pharmacokinetics4-7
Drug |
Half-life (hr) |
Protein binding (%) |
Renal elimination (%) |
Dabigatran |
12-17 |
35 |
80a |
Rivaroxaban |
5-9 |
92-95 |
36 |
Apixaban |
12 |
87 |
27 |
Edoxaban |
10-14 |
55 |
50 |
DOAC = direct-acting oral anticoagulant
aDabigatran is the only DOAC known to be dialyzable (rivaroxaban, apixaban, and edoxaban are not removed by dialysis).
Bleeding is a potential complication associated with the use of all anticoagulants, including DOACs. This e-pub provides a summary of bleeding risk associated with DOACs, reversal options for DOACs, recent clinical evidence of the safety and efficacy of specific pharmacologic reversal agents for DOACs, and recommended reversal strategies from authoritative evidence-based protocols and guidelines. These evidence-based guidelines should be taken into consideration in developing institutional protocols and guidelines for the use of DOAC reversal agents to reduce practice variation and optimize patient outcomes.
DOAC Prescribing Patterns
Oral anticoagulant use in ambulatory and long-term care patients has increased in the United States in recent years in part because of the aging of the population.10,11 The proportion of patients for whom DOACs are prescribed has increased rapidly in the time since the first DOAC (dabigatran) was approved by the Food and Drug Administration (FDA) in 2010, especially among patients with nonvalvular atrial fibrillation because of changes in treatment guidelines that have increased patient eligibility.10-12 Oral anticoagulant use in outpatient office visits for atrial fibrillation nearly doubled between 2009 and 2014.10 In a retrospective analysis, the percentage of Medicare claims for DOACs among beneficiaries receiving oral anticoagulants for atrial fibrillation in U.S. nursing homes increased from less than 5% at the end of 2011 to 48% at the end of 2016.11
In 2014, rivaroxaban (the first oral direct factor Xa [FXa] inhibitor, introduced in 2011) was the most commonly prescribed DOAC for atrial fibrillation in ambulatory care patients in the United States, accounting for almost half of prescriptions, followed by apixaban and dabigatran with approximately one in four prescriptions for each of these two drugs.10 Apixaban (an oral direct FXa inhibitor introduced in 2012) is now the most commonly prescribed DOAC in ambulatory care and nursing home patients with atrial fibrillation.11,12
Scenarios for DOAC Reversal
The anticoagulant effects of DOACs dissipate quickly after the drugs are discontinued because of their short half-lives.16 Intervention is sometimes needed to hasten the reduction in (i.e., provide reversal of) anticoagulant effects for patients with spontaneous or traumatic life-threatening or uncontrolled bleeding or an urgent (i.e., within 1 hour) need for surgery or another invasive procedure.9 Bleeding is defined in the American College of Cardiology (ACC) Expert Consensus Decision Pathway (ECDP) on Management of Bleeding in Patients on Oral Anticoagulants as major (i.e., life-threatening) if it occurs at a critical site that compromises organ function, causes hemodynamic instability, or is overt and causes a substantial decrease in hemoglobin or requires transfusion (Table 2).17
Table 2. American College of Cardiology Definition of Major Bleeding17
Criteria Defining Major Bleedinga |
Bleeding at a critical site
Bleeding that causes hemodynamic instability
Bleeding that is overt and causes a decrease in hemoglobin by ≥2 g/dL or requires the administration of ≥2 units of packed red blood cells |
DBP = diastolic blood pressure; ICH = intracranial hemorrhage; SBP = systolic blood pressure
aDefined as one or more of the criteria in this table.
The need for DOAC reversal in patients undergoing surgery or other invasive procedures often depends on the urgency of the procedure. Reversal may be indicated for patients requiring life-saving procedures within 1 hour but not for patients for whom the procedure is less urgent (i.e., procedures that can be performed on an expedited basis within 1-24 hours or even later).9
Although routine laboratory monitoring is not required for patients receiving DOACs, assays that provide useful information about anticoagulation status are available for patients receiving DOACs who experience life-threatening or uncontrolled bleeding or require urgent surgery (Table 3). The use of these assays could avoid the unnecessary use of DOAC reversal strategies. Assays for quantifying DOAC levels (e.g., liquid chromatography-tandem mass spectrometry, dilute thrombin time [dTT], ecarin clotting time [ECT], ecarin chromogenic assay, chromogenic anti-FXa assays) are not widely available, which is an important limitation when managing patients with spontaneous or traumatic bleeding or an urgent need for surgery.17 More conventional assays that are widely available, such as thrombin time (TT), prothrombin time (PT), and activated partial thromboplastin time (aPTT), may be used for qualitative assessment of the presence of clinically-relevant DOAC levels when quantitative methods are not feasible. Clinically-relevant DOAC levels are those that may contribute to bleeding or a risk for bleeding associated with surgery. Reversal might be considered for patients with major bleeding and a DOAC level exceeding 50 ng/mL and patients requiring an invasive procedure with a high risk of bleeding and a DOAC level exceeding 30 ng/mL.
Table 3. Laboratory Assays for DOACs17
Type of Assay |
Dabigatran |
Rivaroxaban, Apixaban, and Edoxaban |
Quantitative |
LC-MS/MSa |
LC-MS/MSa |
Qualitative |
TTc |
UFH or LMWH anti-FXae |
aPTT = activated partial thromboplastin time; DOAC = direct-acting oral anticoagulant; dTT = dilute thrombin time; ECA = ecarin chromogenic assay; ECT = ecarin clotting time; FXa = factor Xa; LC-MS/MS = liquid chromatography-tandem mass spectrometry; LMWH = low molecular weight heparin; PT = prothrombin time; TT = thrombin time; UFH = unfractionated heparin
aLC-MS/MS, dTT, ECT, ECA, and anti-FXa assays are not widely available.
bThe anti-FXa assay is the preferred test for quantifying rivaroxaban, apixaban, and edoxaban levels. The assay must be calibrated with the specific DOAC.
cA normal TT excludes clinically-relevant dabigatran levels but a prolonged TT does not differentiate between clinically significant and insignificant dabigatran levels.
dA normal aPTT usually excludes clinically-relevant dabigatran levels, and a prolonged aPTT suggests on-therapy or supratherapeutic dabigatran levels.
eA UFH or LMWH anti-FXa level below the lower limit of quantitation probably excludes clinically-relevant levels of rivaroxaban, apixaban, and edoxaban.
fA normal PT or aPTT value does not exclude clinically-relevant levels of rivaroxaban, apixaban, and edoxaban. A prolonged PT suggests on-therapy or supratherapeutic drug levels.
If a quantitative assay for dabigatran is not available, a normal TT excludes the presence of clinically-relevant dabigatran levels, although a prolonged TT does not differentiate between clinically significant and insignificant dabigatran levels. A normal aPTT usually excludes clinically-relevant dabigatran levels, and a prolonged aPTT suggests on-therapy or supratherapeutic dabigatran levels.
Use of a chromogenic anti-FXa assay calibrated with the direct FXa inhibitor is preferred to quantify levels of rivaroxaban, apixaban, and edoxaban. An anti-FXa assay calibrated with an unfractionated heparin (UFH) or low molecular weight heparin (LMWH) standard may be used instead of an assay calibrated with the FXa inhibitor to exclude the presence of clinically-important levels of the drug.
Nonpharmacologic Approaches
Withholding the DOAC may suffice to reduce anticoagulant activity in patients with non-major bleeding or a need for surgery that is not urgent or life-saving (e.g., a procedure not required within the next 24 hours).8,9 In patients preparing to undergo surgery, the duration required for withholding the DOAC depends on the DOAC, renal function, and risk for bleeding associated with the procedure.8 Additional interventions beyond withholding the DOAC often are required for patients with major bleeding or an urgent need for surgery within 1 hour.
Activated charcoal may be given orally to reduce the absorption of the DOAC if less than 2-4 hours have elapsed since the last DOAC dose was taken.17 In theory, hemodialysis is potentially useful for removing dabigatran because the drug relies on the kidneys for elimination, and its protein binding is low. Hemodialysis is not useful for removal of oral direct FXa inhibitors because of the high protein binding of these drugs.16 In practice, hemodialysis is seldom used to reverse dabigatran because a specific reversal agent (idarucizumab) is now available, and inserting a dialysis catheter is problematic for patients with major bleeding.
Nonspecific Pharmacologic Strategies
Various nonspecific pharmacologic interventions (e.g., fresh frozen plasma [FFP], recombinant factor VIIa) have been used to reverse DOACs based largely on in vitro data (i.e., hematologic biomarkers) and limited clinical data from patients with DOAC-related bleeding. Some of these interventions have fallen out of favor because of their limitations, a lack of data and recommendations in current guidelines for DOAC reversal supporting their use, and the introduction of specific pharmacologic interventions that are safe and effective. For example, FFP is seldom used now because it requires time for thawing, which is a potential problem for patients with life-threatening bleeding or an urgent need for surgery, and it involves the administration of a large volume of fluid.9
Concentrated clotting factor products, including four-factor prothrombin complex concentrate (PCC4) products and activated prothrombin complex concentrate (aPCC, also known as factor VIII inhibitor bypassing activity [FEIBA]), are among the nonspecific therapies that have been used to reverse DOACs. Four-factor PCC products contain the four vitamin K-dependent clotting factors II, VII, IX, and X in an inactivated form. Activated PCC contains clotting factor VII in an activated form and clotting factors II, IX, and X primarily in an inactivated form.9 These therapies replenish the clotting factors inhibited by DOACs but they do not directly affect the DOAC.18 A risk for thrombosis is associated with the use of both PCC4 products and aPCC products.
Retrospective reviews of the use of PCC4 for reversing the anticoagulant effects of oral direct FXa inhibitors in patients with major bleeding (mostly rivaroxaban and apixaban in patients with ICH) suggest that this intervention is effective for controlling bleeding with a low risk for thromboembolic events.19-21 Piran and colleagues conducted a meta-analysis of studies of PCC4 use for the reversal of oral direct FXa inhibitors in patients with major bleeding.22 The researchers found it impossible to determine whether this intervention in combination with discontinuation of the FXa inhibitor and provision of supportive care was safer or more effective than discontinuing the FXa inhibitor and providing supportive care alone because of the lack of a control group in the studies included in the analysis and the low quality of evidence. Randomized controlled studies are needed.
Clinical data for the use of PCC4 to reverse the anticoagulant effects of edoxaban are even more limited than for rivaroxaban and apixaban, although in vitro data are available. The efficacy and safety of PCC4 for reversing the effects of edoxaban on bleeding duration and biomarkers of bleeding have been demonstrated in healthy individuals.23
One PCC4 product is available in the U.S., and it is a lyophilized powder for reconstitution.24 It is provided as a single-use vial of either 500 or 1000 units (defined by clotting factor IX content). The range of clotting factor IX units per vial is 400-620 units for the 500-unit vial and 800-1240 units for the 1000-unit vial, with the actual units of potency for clotting factor IX stated on the vial and carton, along with actual units of potency for the other clotting factors. Dosing is highly variable based on body weight as defined in the prescribing information or as a fixed dose of 2000 units.24-26
Reports of the clinical efficacy of aPCC for DOAC reversal are also limited. Schulman and colleagues reported that the administration of aPCC was effective for controlling life-threatening dabigatran-related bleeding in four patients with atrial fibrillation.27 In a case series of six patients with spontaneous ICH while taking rivaroxaban, apixaban, or dabigatran for atrial fibrillation, none of the patients experienced ICH expansion or hemorrhagic or thrombotic complications when aPCC was used for reversal.28 A retrospective analysis of 64 patients treated with aPCC for DOAC-related major bleeding suggested that low (<20 units/kg) to moderate (20-30 units/kg) doses, with the option for a repeat dose, is an effective management strategy.29 Research is under way to clarify the most effective aPCC dosage for DOAC reversal.
Idarucizumab
The introduction of pharmacologic agents that specifically target DOACs changed the approach used to manage patients with major bleeding or an urgent need for surgery. These agents bind to and reverse the anticoagulant effects of DOACs, unlike concentrated clotting factor products, which replenish clotting factors.8
Idarucizumab was approved by FDA in 2015 for reversal of the anticoagulant effects of dabigatran in patients with life-threatening or uncontrolled bleeding or in need of emergency surgery or urgent procedures.30 The drug is not useful for reversing the anticoagulant effects of oral direct FXa inhibitors. Idarucizumab is a humanized monoclonal antibody fragment (Fab) with an approximately 350-fold higher affinity for dabigatran than the affinity of dabigatran for thrombin.31 The reversal agent binds to and neutralizes the anticoagulant effects of unbound and thrombin-bound dabigatran. Idarucizumab does not bind to thrombin, activate platelets, or convert fibrinogen to fibrin.
Idarucizumab is available as a 2.5-g/50-mL sterile solution in single-dose vials.30 The recommended dose is 5 g (i.e., two vials) given as two consecutive intravenous (IV) injections or infusions. If the drug is administered through an established IV line, the line should be flushed with 0.9% sodium chloride before and after administration.
Idarucizumab vials should be stored in a refrigerator (i.e., 2°C to 8°C, or 36°F to 46°F) and protected from light, although they may be stored for up to 48 hours at room temperature (i.e., 25°C or 77°F).30 The vials should be used within 6 hours if stored at room temperature without protection from light. The vials should not be shaken or allowed to freeze.
Approval of idarucizumab by FDA was based largely on the findings from the RE-VERSal Effects of idarucizumab on Active Dabigatran (RE-VERSE AD) trial of 503 patients treated with dabigatran who presented with life-threatening or uncontrolled bleeding (group A) or required emergency surgery or an urgent procedure (group B).30,32-34 The maximum percentage reversal of the anticoagulant effects of dabigatran within 4 hours after idarucizumab administration measured using the dTT or ECT was the primary outcome, and restoration of hemostasis was a secondary outcome. Elevated dTT and ECT values were normalized within minutes after administration of the reversal agent in most patients. The median maximum percentage reversal of the anticoagulant effects of dabigatran within 4 hours after idarucizumab administration was 100% (i.e., reversal was complete for most patients). Nearly half of the patients in group A presented with GI bleeding, and another third presented with ICH. Assessing the time to cessation of bleeding after idarucizumab administration was not feasible in patients with ICH. Hemostasis was achieved within 24 hours in group A patients with non-ICH bleeding, with a median time to bleeding cessation of 2.5 hours. In group B, the median time between the first infusion of idarucizumab and initiation of the surgical procedure was 1.6 hours. Periprocedural hemostasis was normal in most (93%) patients. Thrombotic events were reported within 90 days by 6.3% of patients in group A and 7.4% of patients in group B. The 90-day mortality rate was similar in the two groups (19%). Anti-idarucizumab antibodies were detected in 5.6% of patients.
Limited data are available from the use of more than one idarucizumab 5-g dose for dabigatran reversal in patients with recurrent or persistent bleeding or a need for emergency surgery and elevated coagulation parameters despite the use of one dose. Eight of the 503 RE-VERSE AD trial participants received more than one idarucizumab 5-g dose because of recurrent bleeding, a need for a second emergency surgery, or bleeding after a first emergency surgery.30,32
Andexanet Alfa
Andexanet alfa (also known as coagulation factor Xa [recombinant], inactivated-zhzo) was approved by FDA in 2018 for reversal of the anticoagulant effects from rivaroxaban or apixaban in patients with life-threatening or uncontrolled bleeding.35 This approval was under the agency’s accelerated approval pathway and based on data demonstrating a reduction from baseline in anti-FXa activity in healthy volunteers treated with apixaban or rivaroxaban (anti-FXa activity is a measure of the anticoagulant activity of the FXa inhibitor). The effect of the drug in restoring hemostasis was not established at the time of FDA approval. Andexanet alfa is not approved for reversing the anticoagulant effects of edoxaban, although a reduction in anti-FXa activity has been demonstrated with andexanet alfa in healthy volunteers treated with edoxaban.17 The off-label use of andexanet alfa to reverse the anticoagulant effects of edoxaban is recommended in some treatment guidelines.
Andexanet alfa is a recombinant modified human FXa protein that serves as a decoy for and binds to oral direct FXa inhibitors.8,34 It does not convert prothrombin (factor II) to thrombin (factor IIa) in the final common pathway of the clotting cascade (Figure).8 Andexanet alfa does not interfere with activation of native factor X by tissue factor or factor VIIa (i.e., endogenous hemostasis).34
Andexanet alfa is available as a sterile lyophilized cake or powder in single-use vials that contain 200 mg.35 Unopened vials should be stored under refrigeration and not allowed to freeze.
According to the andexanet alfa prescribing information, one of two andexanet alfa dosing regimens (low-dose and high-dose) should be used depending on whether the patient took rivaroxaban or apixaban, the last dose, and the time elapsed since it was taken (Tables 4 and 5).35 The low-dose regimen should be used for patients who took rivaroxaban or apixaban 8 or more hours ago regardless of the dose, patients whose last rivaroxaban dose was 10 mg or less regardless of the timing, and patients whose last apixaban dose was 5 mg or less regardless of the timing. High-dose andexanet alfa should be used for patients whose last rivaroxaban dose was more than 10 mg or unknown taken less than 8 hours ago or at an unknown time, and for patients whose last apixaban dose was more than 5 mg or unknown taken less than 8 hours ago or at an unknown time.
Table 4. Andexanet Alfa Dosing35
Oral Direct FXa Inhibitor and Last Dose |
Time Elapsed Since Last Dose <8 hr or Unknown |
Time Elapsed Since Last Dose ≥8 hr |
Rivaroxaban ≤10 mg |
Low-dose regimena |
Low-dose regimena |
Rivaroxaban >10 mg or unknown |
High-dose regimenb |
Low-dose regimena |
Apixaban ≤5 mg |
Low-dose regimena |
Low-dose regimena |
Apixaban >5 mg or unknown |
High-dose regimenb |
Low-dose regimena |
FXa = factor Xa
aThe low-dose regimen involves 400 mg as an IV bolus followed by 4 mg/min for up to 120 min (i.e., 480 mg by IV infusion) and requires five 200-mg vials.
bThe high-dose regimen involves 800 mg as an IV bolus followed by 8 mg/min for up to 120 min (i.e., 960 mg by IV infusion) and requires nine 200-mg vials.
Table 5. Andexanet Alfa Dosing Regimens35
Regimen |
No. of 200-mg Vials |
Initial IV Bolusa |
Follow-On IV Infusionb |
Low-dose |
5 |
400 mg |
4 mg/min for up to 120 min (480 mg) |
High-dose |
9 |
800 mg |
8 mg/min for up to 120 min (960 mg) |
IV = intravenous
aA target rate of 30 mg/min should be used for the initial IV bolus dose.
bThe follow-on infusion should begin within 2 min after the initial IV bolus dose.
A target rate of 30 mg/min should be used for an initial IV bolus dose of andexanet alfa followed within 2 minutes by an IV infusion for up to 120 minutes.35 The low-dose regimen involves 400 mg as an IV bolus followed by 4 mg/min for up to 120 min (i.e., 480 mg by IV infusion) and requires five 200-mg vials. The high-dose regimen involves 800 mg as an IV bolus followed by 8 mg/min for up to 120 min (i.e., 960 mg by IV infusion) and requires nine 200-mg vials.
The contents of each andexanet alfa vial should be reconstituted by slowly injecting 20 mL of sterile water for injection toward the inside wall of the vial to minimize foaming.35 The vial should be gently swirled (not shaken) until the cake or powder is completely dissolved, which typically takes 3-5 minutes. The vial should be discarded if dissolution is incomplete. The solution should be transferred to an IV bag in accordance with the instructions in the prescribing information.
Because the andexanet alfa reconstitution process takes time and five or nine vials are needed for each patient, all of the required vials should be reconstituted at the same time. Reconstituted andexanet alfa in vials is stable for up to 8 hours at room temperature and 24 hours in a refrigerator.35
Approval by FDA of andexanet alfa was based largely on the findings from two large, prospective, randomized, placebo-controlled studies of 145 healthy older (median age 57 years) volunteers treated with apixaban 5 mg orally twice daily for 3.5 days (ANNEXA-A) or rivaroxaban 20 mg orally once daily for 4 days (ANNEXA-R).35,36 The low-dose andexanet alfa regimen or placebo was given 3 hours after the last apixaban dose, and the high-dose regimen or placebo was given 4 hours after the last rivaroxaban dose. The mean percentage change from baseline in anti-FXa activity at the nadir (i.e., the lowest value within 5 minutes after the end of the andexanet alfa infusion) was used as the primary outcome. In ANNEXA-A, the reduction in anti-FXa activity was significantly greater with andexanet alfa (94%) than placebo (21%, p <0.001). Similarly, in ANNEXA-R, the reduction in anti-FXa activity was significantly greater with andexanet alfa (92%) than placebo (18%, p <0.001). In both studies, the thrombin generation that had been inhibited by treatment with apixaban or rivaroxaban was fully restored within 2 to 5 minutes after administration of the andexanet alfa IV bolus doses. No thrombotic events or other serious or severe adverse events were reported in either study.
The hemostatic efficacy and impact on anti-FXa activity of andexanet alfa were evaluated in ANNEXA-4, a prospective, open-label, single-group study of 352 patients (mean age 77 years) with acute major bleeding within 18 hours after receipt of an oral direct FXa inhibitor and a baseline anti-FXa activity of at least 75 ng/mL.37 Approximately two thirds of the study participants presented with ICH, and one in four had GI bleeding. The observed reduction from baseline in anti-FXa activity until the nadir was similar to that observed with andexanet alfa in the ANNEXA-A and ANNEXA-R trials. Excellent or good hemostasis within 12 hours after the end of the andexanet alfa infusion was reported by 204 (82%) of the 249 evaluable ANNEXA-4 patients. The reduction from baseline in anti-FXa activity was modestly correlated with hemostatic efficacy only in patients with ICH. In a 30-day follow-up period, the incidence of death was 14% and the incidence of thrombotic events was 10%. Neutralizing antibodies to andexanet alfa did not form. The number of ANNEXA-4 study participants treated with edoxaban was small (four in the efficacy analysis), so additional clinical experience with the use of andexanet alfa for reversal of the anticoagulant effects of this oral direct FXa inhibitor is needed.
The safety and efficacy of andexanet alfa (dosed in accordance with the prescribing information) and PCC4 (25-50 units/kg, not to exceed 5000 units) were compared in a retrospective analysis by Barra and colleagues of a case series of 29 consecutive patients (median age 73 years) with rivaroxaban- or apixaban-related ICH.38 The median time elapsed between ordering and administering the reversal agent was 1.1 hours for andexanet alfa and 0.5 hours for PCC4. Excellent or good hemostasis was achieved in 16 (89%) of the 18 andexanet alfa-treated patients and 6 (60%) of the 10 evaluable PCC4-treated patients. A good functional outcome at the time of hospital discharge (defined as a Glasgow Outcome Score >3) was reported for 10 (56%) of the 18 andexanet alfa-treated patients and 1 (9%) of the 11 PCC4-treated patients, although the median Glasgow Coma Scale (GCS) score on admission was higher in the andexanet alfa group than the PCC4 group (15 and 10, respectively) reflecting less severe injury. The lengths of stay in the hospital and intensive care unit (ICU) were similar in the two groups. New thrombotic events were reported within 30 days after receipt of the reversal agent by 3 (17%) of 18 andexanet alfa-treated patients and 1 (9%) of 11 PCC4-treated patients. The median cost of therapy per patient was $29,970 for andexanet alfa and $6925 for PCC4.
In another retrospective analysis of 44 consecutive patients (median age 79 years) with apixaban- or rivaroxaban-related ICH, no significant differences between andexanet alfa (dosed in accordance with the prescribing information) and PCC4 (25 units/kg, not to exceed 2500 units) were observed in neuroimaging stability (i.e., the amount of blood on computed tomographic scans reflecting hemostasis) 6 hours and 24 hours after reversal agent administration, good functional outcome at the time of hospital discharge (defined as a Modified Rankin score ≤3), or thromboembolic events.39 There were no significant differences between the two treatments in ICU or hospital length of stay. The median GCS score at the time of admission was the same (14) in the two groups. Prospective studies comparing the efficacy and safety of these interventions for FXa inhibitor reversal are needed.
Ciraparantag
Ciraparantag (formerly known as PER977 and aripazine) is a small synthetic, water-soluble, cationic molecule that forms noncovalent hydrogen bonds with oral direct FXa inhibitors, direct thrombin inhibitors (e.g., dabigatran), UFH, LMWH, and fondaparinux and interferes with their anticoagulant effects.8,34 In phase 2 clinical trials, rapid and sustained reversal of the anticoagulant effects of apixaban, rivaroxaban, and edoxaban was demonstrated with a single IV dose of ciraparantag using whole blood clotting times in healthy adults.40,41 The reversal agent does not appear to have prothrombotic effects.40 Mild, transient sensations of warmth (i.e., hot flashes or flushing) were the most commonly reported adverse events. Phase 3 clinical trials are planned. A broad spectrum of reversal activity, rapid onset of action, and administration as a single dose are among the potential advantages of ciraparantag.34
Key Guidelines
Practice guidelines addressing DOAC reversal are available from various organizations, including the ACC, American College of Emergency Physicians, and Anticoagulation Forum (a North American organization of anticoagulation providers).17,18,42 These guidelines are consistent in recommending the first-line use of idarucizumab for dabigatran reversal and andexanet alfa for FXa inhibitor reversal, with use of PCC4 or aPCC as an alternative strategy when the specific pharmacologic reversal agent is not available, although there are minor differences in the recommended approach to reversal (Table 6). Guidelines from other authoritative sources are listed in the Appendix.
Table 6. Recommendations in Key Practice Guidelines for Use of DOAC Reversal Agents in Patients with Major Bleeding or an Urgent Need for Surgery17,18,24,30,35,42,43,a
Practice Guideline and DOAC |
First-line |
Alternative if First-line Not Available |
American College of Cardiology |
||
Dabigatran |
Idarucizumab 5 gb |
PCCc or aPCCc |
Rivaroxaban and apixaban |
Andexanet alfad |
PCCc or aPCCc |
Edoxaban |
High-dose andexanet alfae |
PCCc or aPCCc |
American College of Emergency Physicians |
||
Dabigatran |
Idarucizumab 5 g if <8-12 hr since last dabigatran doseb |
PCC4 (preferably) 10-100 units/kg or aPCC 10-100 units/kg |
Rivaroxaban, apixaban, and edoxaban |
Andexanet alfad if <18 hr since last rivaroxaban or apixaban dose or 10-14 hr since last edoxaban dosee |
PCC4 (preferably) 10-100 units/kg or aPCC 10-100 units/kg |
Anticoagulation Forum |
||
Dabigatran |
Idarucizumab 5 gb |
aPCC 50 units/kg |
Rivaroxaban and apixaban |
Andexanet alfad |
PCC4 2000 units |
Edoxaban |
High-dose andexanet alfae |
PCC4 2000 units |
aPCC = activated prothrombin complex concentrate; DOAC = direct-acting oral anticoagulant; PCC = prothrombin complex concentrate; PCC4 = four-factor prothrombin complex concentrate
aAll therapies are administered intravenously.
bThe guideline-recommended dosing for idarucizumab is consistent with the FDA-approved prescribing information, which supports administration of an additional 5-g dose in patients with elevated coagulation parameters and reappearance of clinically-relevant bleeding or requiring a second emergency surgery or urgent procedure based on limited data.
cThe guideline-recommended dosing for PCC and aPCC is consistent with the FDA-approved prescribing information.
dThe guideline-recommended dosing for andexanet alfa is consistent with the FDA-approved prescribing information.
eThe use of andexanet alfa for reversal of edoxaban is considered an off-label use of the reversal agent.
The recommended dosing of specific pharmacologic reversal agents in current practice guidelines generally is consistent with the FDA-approved prescribing information for these agents, although the use of high-dose andexanet alfa for the reversal of edoxaban is an off-label use. The Anticoagulation Forum recommends the use of fixed doses of PCC4 2000 units instead of weight-based doses for the reversal of FXa inhibitors because of the availability of clinical data demonstrating the hemostatic efficacy of this fixed dose in patients with major bleeding from oral direct FXa inhibitors, greater simplicity for ordering, and lower cost.39
Practice Variation
There is considerable variability in the approach used for DOAC reversal in clinical practice despite the availability of treatment guidelines from authoritative sources. The Anticoagulation Reversal Survey (ARES) was conducted in early 2019 to assess current practices in the United States for reversing anticoagulant-associated life-threatening bleeding.44 A cross-sectional analysis of the responses from 281 critical care and emergency medicine pharmacists revealed that most hospitals (73%) use PCC4 for oral direct FXa inhibitor reversal, with another 13% of hospitals using andexanet alfa for this purpose, despite the use of a formal institutional protocol or guideline for FXa inhibitor reversal at most (80%) hospitals. The rates of use of andexanet alfa were significantly higher in teaching hospitals and hospitals with a dedicated emergency department (ED) pharmacist compared with nonteaching hospitals and facilities without an ED pharmacist, respectively. The most commonly used PCC dose for FXa inhibitor reversal reported by approximately half of survey respondents was 50 units/kg. The majority of hospitals (96%) use idarucizumab for dabigatran reversal. The most common PCC dose used for dabigatran reversal by roughly one third of respondents was 25 units/kg. The ARES researchers speculated that the use of PCC products despite guideline recommendations for the preferential use of specific reversal agents might be attributed in part to supply limitations, cost differences, and a lack of clinician experience with use of the newer agents.
Evidence-based institutional protocols and guidelines for the use of DOAC reversal agents and management of patients with major bleeding or an urgent need for surgery are needed to reduce practice variation and provide consistent high-quality care. Approved protocols and evidence-based practice guidelines for reversal of anticoagulation and management of bleeding events related to each anticoagulant medication is a 2021 National Patient Safety Goal (03.05.01) of The Joint Commission.45
A wide variety of definitions for major or life-threatening bleeding have been used in clinical trials.18 The use of a consistent definition of major or life-threatening bleeding is needed in institutional protocols and guidelines to determine when to use DOAC reversal strategies and avoid the unnecessary use of these strategies.
Conclusion
The use of DOACs has increased in the United States, and the risk of bleeding from DOAC use is a safety concern. Reversal of the anticoagulant effects of DOACs may be needed for patients with life-threatening or uncontrolled bleeding or an urgent need for surgery. Evidence-based guidelines for DOAC reversal are available from authoritative sources to guide treatment decisions for these patients. These guidelines should be the basis for DOAC reversal strategies outlined in institutional protocols and guidelines. Efforts to standardize strategies have the potential to optimize patient outcomes.
References
- Kalus JS. Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health-Syst Pharm. 2013; 70(suppl 1):S12-21. doi:10.2146/ajhp130041. https://pubmed.ncbi.nlm.nih.gov/23640528/ (accessed 2021 May 5).
- Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383:955-62. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(13)62343-0 (accessed 2021 May 5).
- Kearon C, Akl EA, Ornelas J et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016; 149:315-52. doi.org/10.1016/j.chest.2015.11.026. https://www.sciencedirect.com/science/article/pii/S0012369215003359 (accessed 2021 May 5).
- Pradaxa (dabigatran etexilate) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2021 Apr. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf (accessed 2021 May 5).
- Xarelto (rivaroxaban) prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2021 Jan. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf (accessed 2021 May 5).
- Eliquis (apixaban) prescribing information. Princeton, NJ and New York, NY: Bristol-Myers Squibb Company and Pfizer Inc; 2021 Mar. https://packageinserts.bms.com/pi/pi_eliquis.pdf (accessed 2021 May 5).
- Savaysa (edoxaban) prescribing information. Basking Ridge, NJ: Daiichi Sankyo, Inc.; 2021 Mar. https://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true (accessed 2021 May 5).
- Gulseth MP. Overview of direct oral anticoagulant therapy reversal. Am J Health-Syst Pharm. 2016; 73(suppl 2):S5-13. doi:10.2146/ajhp150966. https://academic.oup.com/ajhp/article-abstract/73/10_Supplement_2/s5/5101533?redirectedFrom=PDF (accessed 2021 May 5).
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ III, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health-Syst Pharm. 2013; 70:1914-29. doi: 10.2146/ajhp130243. https://pubmed.ncbi.nlm.nih.gov/24128967/ (accessed 2021 May 5).
- Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015; 128:1300-5.e2. doi:10.1016/j.amjmed.2015.05.044. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4658248/pdf/nihms707484.pdf (accessed 2021 May 5).
- Alcusky M, McManus DD, Hume AL et al. Changes in anticoagulant utilization among United States nursing home residents with atrial fibrillation from 2011 to 2016. J Am Heart Assoc. 2019; 8:e012023. doi:10.1161/JAHA.119.012023. https://www.ahajournals.org/doi/epub/10.1161/JAHA.119.012023 (accessed 2021 May 5).
- Kattoor AJ, Pothineni NV, Goel A et al. Prescription patterns and outcomes of patients with atrial fibrillation treated with direct oral anticoagulants and warfarin: a real-world analysis. J Cardiovasc Pharmacol Ther. 2019; 24:428-34. doi:10.1177/1074248419841634. https://pubmed.ncbi.nlm.nih.gov/31035795/ (accessed 2021 May 5).
- Milling TJ Jr, Frontera JA. Exploring indications for the use of direct oral anticoagulants and the associated risks of major bleeding. Am J Manag Care. 2017; 23(suppl 4):S67-S80. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5568002/ (accessed 2021 May 5).
- Held C, Hylek EM, Alexander JH et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015; 36:1264-72. doi:10.1093/eurheartj/ehu463. https://academic.oup.com/eurheartj/article/36/20/1264/2293215 (accessed 2021 May 5).
- Deitelzweig S, Neuman WR, Lingohr-Smith M et al. Incremental economic burden associated with major bleeding among atrial fibrillation patients treated with factor Xa inhibitors. J Med Econ. 2017; 20:1217-23. doi.org/10.1080/13696998.2017.1362412. https://www.tandfonline.com/doi/full/10.1080/13696998.2017.1362412 (accessed 2021 May 5).
- Frontera JA, Lewin JJ III, Rabinstein AA et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016; 24:6-46. doi:10.1007/s12028-015-0222-x. https://pubmed.ncbi.nlm.nih.gov/26714677/ (accessed 2021 May 5).
- Tomaselli GF, Mahaffey KW, Cuker A et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020; 76:594-622. doi: 10.1016/j.jacc.2020.04.053. https://pubmed.ncbi.nlm.nih.gov/32680646/ (accessed 2021 May 5).
- Baugh CW, Levine M, Cornutt D et al. Anticoagulant reversal strategies in the emergency department setting: recommendations of a multidisciplinary expert panel. Ann Emerg Med. 2019; 76:470-85. doi: 10.1016/j.annemergmed.2019.09.001. https://pubmed.ncbi.nlm.nih.gov/31732375/ (accessed 2021 May 5).
- Panos NG, Cook AM, John S et al. Factor Xa inhibitor-related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020; 141:1681-9. doi:10.1161/CIRCULATIONAHA.120.045769. https://pubmed.ncbi.nlm.nih.gov/32264698/ (accessed 2021 May 5).
- Smith MN, Deloney L, Carter C et al. Safety, efficacy, and cost of four-factor prothrombin complex concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study. J Thromb Thrombolysis. 2019; 48:250-5. doi:10.1007/s11239-019-01846-5. https://pubmed.ncbi.nlm.nih.gov/30941571/ (accessed 2021 May 5).
- Grandhi R, Newman WC, Zhang X et al. Administration of 4-factor prothrombin complex concentrate as an antidote for intracranial bleeding in patients taking direct factor Xa inhibitors. World Neurosurg. 2015; 84:1956-61. doi: 10.1016/j.wneu.2015.08.042. https://pubmed.ncbi.nlm.nih.gov/26341438/ (accessed 2021 May 5).
- Piran S, Khatib R, Schulman S et al. Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis. Blood Adv. 2019; 3:158-67. doi:10.1182/bloodadvances.2018024133. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6341194/pdf/advances024133.pdf (accessed 2021 May 5).
- Zahir H, Brown KS, Vandell AG et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation. 2015; 131:82-90. doi:10.1161/CIRCULATIONAHA.114.013445. https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.114.013445 (accessed 2021 May 5).
- Kcentra (prothrombin complex concentrate, human) prescribing information. Kankakee, IL: CSL Behring LLC; 2018 Oct. https://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf (accessed 2021 May 5).
- Schulman S, Gross PL, Ritchie B et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018; 118:842-51. doi: 10.1055/s-0038-1636541.
- Majeed A, Agren A, Holmstrom M et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017; 130:1706-12. doi: 10.1182/blood-2017-05-782060. https://ashpublications.org/blood/article/130/15/1706/36377/Management-of-rivaroxaban-or-apixaban-associated (accessed 2021 May 5).
- Schulman S, Ritchie B, Goy JK et al. Activated prothrombin complex concentrate for dabigatran-associated bleeding. Br J Haematol. 2013; 164:308-10. doi:10.1111/bjh.12620. https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.12620 (accessed 2021 May 5).
- Dibu JR, Weimer JM, Ahrens C et al. The role of FEIBA in reversing novel oral anticoagulants in intracerebral hemorrhage. Neurocrit Care. 2016; 24:413-9. doi:10.1007/s12028-015-0213-y. https://link.springer.com/article/10.1007/s12028-015-0213-y (accessed 2021 May 5).
- Dager WE, Roberts AJ, Nishijima DK. Effect of low and moderate dose FEIBA to reverse major bleeding in patients on direct oral anticoagulants. Thromb Res. 2019; 173:71-6. doi:10.1016/j.thromres.2018.11.009. https://pubmed.ncbi.nlm.nih.gov/30476716/ (accessed 2021 May 5).
- Praxbind (idarucizumab) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2018 Apr. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf (accessed 2021 May 5).
- Eikelboom JW, Quinlan DJ, van Ryn J, Weitz JI. Idarucizumab: the antidote for reversal of dabigatran. Circulation. 2015; 132:2412-22. doi:10.1161/CIRCULATIONAHA.115.019628. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.115.019628 (accessed 2021 May 5).
- Pollack CV Jr, Reilly PA, van Ryn J et al. Idarucizumab for dabigatran reversal−full cohort analysis. N Engl J Med. 2017; 377:431-41. doi:10.1056/NEJMoa1707278. https://www.nejm.org/doi/pdf/10.1056/NEJMoa1707278?articleTools=true (accessed 2021 May 5).
- Pollack CV Jr, Reilly PA, Eikelboom J et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015; 373:511-20. doi: 10.1056/NEJMoa1502000. https://www.nejm.org/doi/full/10.1056/nejmoa1502000 (accessed 2021 May 5).
- Smythe MA, Trujillo T, Fanikos J. Reversal agents for use with direct and indirect anticoagulants. Am J Health-Syst Pharm. 2016; 73(suppl 2):S27-48. doi:10.2146/ajhp150959. https://pubmed.ncbi.nlm.nih.gov/27147456/ (accessed 2021 May 5).
- Andexxa (coagulation factor Xa [recombinant], inactivated-zhzo) prescribing information. Boston, MA: Alexion Pharmaceuticals, Inc; 2021 Feb. https://alexion.com/Documents/andexxa_uspi.pdf ( accessed 2021 May 5).
- Siegal DM, Curnutte JT, Connolly SJ et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015; 373:2413-24. doi:10.1056/NEJMoa1510991. https://www.nejm.org/doi/pdf/10.1056/NEJMoa1510991?articleTools=true (accessed 2021 May 5).
- Connolly SJ, Crowther M, Eikelboom JW et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019; 380:1326-35. doi:10.1056/NEJMoa1814051. https://www.nejm.org/doi/pdf/10.1056/NEJMoa1814051?articleTools=true (accessed 2021 May 5).
- Barra ME, Das AS, Hayes BD et al. Evaluation of andexanet alfa and four-factor prothrombin complex concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J Thromb Haemost. 2020; 18:1637-47. doi:10.1111/jth.14838. https://onlinelibrary.wiley.com/doi/abs/10.1111/jth.14838 (accessed 2021 May 5).
- Ammar AA, Ammar MA, Owusu KA et al. Andexanet alfa versus 4-factor prothrombin complex concentrate for reversal of factor Xa inhibitors in intracranial hemorrhage. Neurocrit Care. 2021; Jan 6 [online ahead of print]. doi: 10.1007/s12028-020-01161-5.
- Ansell J, Bakhru S, Villano S, Luo X. Efficacy and safety of ciraparantag in reversing apixaban and rivaroxaban as measured by whole blood clotting time in healthy adults [abstract]. Res Pract Thromb Haemost. 2020; 4 (suppl 1). https://abstracts.isth.org/abstract/efficacy-and-safety-of-ciraparantag-in-reversing-apixaban-and-rivaroxaban-as-measured-by-whole-blood-clotting-time-in-healthy-adults/ (accessed 2021 May 5).
- Ansell JE, Bakhru SH, Laulicht BE et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014; 371:2141-2. doi:10.1056/NEJMc1411800. https://pubmed.ncbi.nlm.nih.gov/25371966/ (accessed 2021 May 5).
- Cuker A, Burnett A, Triller D et al. Reversal of direct oral anticoagulants: guidance from the Anticoagulation Forum. Am J Hematol. 2019; 94:697-709. doi: 10.1002/ajh.25475. https://pubmed.ncbi.nlm.nih.gov/30916798/ (accessed 2021 May 5).
- FEIBA (anti-inhibitor coagulant complex) prescribing information. Lexington, MA: Baxalta US Inc.; 2020 Feb. https://www.shirecontent.com/PI/PDFs/FEIBA_USA_ENG.pdf (accessed 2021 May 5).
- Rowe S, Dietrich S, Hamilton LA. Analysis of anticoagulation reversal survey (ARES). Hospital Practice. 2020; 48:123-7. doi:10.1080/21548331.2020.1753435. https://pubmed.ncbi.nlm.nih.gov/32295428/ (accessed 2021 May 5).
- The Joint Commission. Hospital: 2021 national patient safety goals. https://www.jointcommission.org/standards/national-patient-safety-goals/hospital-national-patient-safety-goals/ (accessed 2021 May 5).
Appendix – Additional Resources
American College of Chest Physicians (ACCP)
Lip GYH, Banerjee A, Boriani G et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018; 154:1121-201. doi: 10.1016/j.chest.2018.07.040. https://pubmed.ncbi.nlm.nih.gov/30144419/ (accessed 2021 May 5).
Ageno W, Buller HR, Falanga A, et al. Managing reversal of direct oral anticoagulants in emergency situations. Anticoagulation Education Task Force White Paper. Thromb Haemost. 2016; 116:1003-10. doi: 10.1160/TH16-05-0363. https://pubmed.ncbi.nlm.nih.gov/27488232/ (accessed 2021 May 5).
American College of Surgeons
Hornor MA, Duane TM, Ehlers AP et al. American College of Surgeons’ guidelines for the perioperative management of antithrombotic medication. J Am Coll Surg. 2018; 227:521-536.e1. doi: 10.1016/j.jamcollsurg.2018.08.183. https://pubmed.ncbi.nlm.nih.gov/30145286/ (accessed 2021 May 5).
American Heart Association
Raval AR, Cigarroa JE, Chung MK et al. Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting. A scientific statement from the American Heart Association. Circulation. 2017; 135:e604-e633. doi:10.1161/CIR.0000000000000477. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5404934/pdf/nihms853705.pdf (accessed 2021 May 5).
American Heart Association/American College of Cardiology/Heart Rhythm Society
January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019; 140:e125-e151. doi: 10.1161/CIR.0000000000000665. https://www.ahajournals.org/doi/epub/10.1161/CIR.0000000000000665 (accessed 2021 May 5).
European Society of Cardiology
Niessner A, Tamargo J Morais J et al. Reversal strategies for non-vitamin K antagonist oral anticoagulants: a critical appraisal of available evidence and recommendations for clinical management-a joint position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2017; 38:1710-6. doi: 10.1093/eurheartj/ehv676. https://pubmed.ncbi.nlm.nih.gov/26705385/ (accessed 2021 May 5).
European Heart Rhythm Association
Steffel J, Verhamme P, Potpara TS et al. ESC Scientific Document Group. The 2018 European Heart Rhythm Association practical guide on the use of the non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018; 39:1330-93. doi: 10.1093/eurheartj/ehy136. https://pubmed.ncbi.nlm.nih.gov/29562325/ (accessed 2021 May 5).
International Society on Thrombosis and Haemostasis
Levy JH, Ageno W, Chan NC et al; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016; 14:623-7. doi: 10.1111/jth.13227. https://onlinelibrary.wiley.com/doi/epdf/10.1111/jth.13227 (accessed 2021 May 5).
Neurocritical Care Society and Society of Critical Care Medicine
Frontera JA, Lewin JJ III, Rabinstein AA et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care
Medicine. Neurocrit Care. 2016; 24:6-46. doi: 10.1007/s12028-015-0222-x. https://pubmed.ncbi.nlm.nih.gov/26714677/ (accessed 2021 May 5).
Planners and Reviewers
The assistance of the planners and reviewers of this educational activity is gratefully acknowledged.
James S. Kalus, PharmD, FASHP
Director of Pharmacy
Henry Ford Health System
Detroit, Michigan
James S. Kalus, PharmD, FASHP, is Director of Pharmacy at Henry Ford Health System in Detroit, Michigan where he is responsible for planning, implementing, and managing all pharmacy services related to patient care including staff training and development, and pharmacy research. Dr. Kalus also serves as Program Director for the postgraduate year one (PGY1) residency at Henry Ford Hospital and is adjunct assistant professor of pharmacy and medicine at Wayne State University. In his research, Dr. Kalus has focused on cardiovascular disease, including the pathophysiology of atrial fibrillation (AF) occurring after cardiac surgery, novel strategies for the treatment and prevention of AF, practice-based research related to anticoagulation and implementation science related to clinical pharmacy services.
Jason R. Vilar, Pharm.D., BCCCP
Neurocritical Care Pharmacy Specialist
AdventHealth Orlando
Orlando, Florida
Jason R. Vilar, Pharm.D., BCCCP, is a neurocritical care pharmacy specialist at AdventHealth Orlando in Orlando, Florida, where he also serves as a co-clinical coordinator for the ASHP-accredited postgraduate year 1 (PGY1) pharmacy residency program. In addition, Dr. Vilar holds an assistant clinical professor position at the University of Florida College of Pharmacy.
Dr. Vilar received his Doctor of Pharmacy degree at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences in Aurora, Colorado. He then completed a PGY1 pharmacy residency at Henry Ford Health System in Detroit, Michigan and a PGY2 critical care pharmacy residency at Wesley Medical Center in Wichita, Kansas.
Dr. Vilar's practice and research has focused on critical care and neurocritical care pharmacotherapy. An active member of the Neurocritical Care Society (NCS) and Society of Critical Care Medicine (SCCM), he has served as a pharmacy committee member for both organizations. He is also a member of ASHP.
Relevant Financial Relationship Disclosure
No relevant financial relationship with an ineligible company.*
*As defined by the Standards of Integrity and Independence definition of ineligible company
Susan R. Dombrowski, B.S.Pharm., M.S., Writer
Carla J. Brink, B.S.Pharm., M.S., CHCP, Staff
CPE Information

The American Society of Health-System Pharmacists is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
Title: Evidence-Based Strategies for Reversing Direct-Acting Oral Anticoagulants
ACPE #: 0204-0000-21-404-H01-P
Launch Date: May 12, 2021
Expiration Date: May 13, 2022
Activity Type: Knowledge-based
CE Credits: 1.0 hour
Activity Fee: Free of charge
Target Audience
This activity was planned to meet the educational needs of pharmacists in hospitals and health systems involved in the management of bleeding associated with the use of direct-acting oral anticoagulant therapy.
Instructions for Processing CE
To receive CE credit, complete the steps below within 60 days of completing the activity.
- Read entire online activity and answer all interactive questions.
- Click "Complete Activity" below to link to complete the evaluation.
- Verify credits were successfully transferred to CPE Monitor before the ACPE 60-day deadline by checking your NABP eProfile account. After the 60-day deadline, ASHP will no longer be able to report credit(s).


